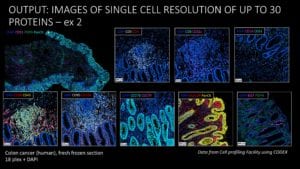
Spatial phenotyping reveals cell type distribution in colorectal cancer
With many away from their labs these past few months, scientists have faced difficulty continuing their research. Several core lab facilities in universities and research institutes have shut down or limited their services, keeping reduced staff on site and working remotely as much as possible.
To get an insider perspective on operating a core lab during the pandemic, we spoke to Kimberly Jordan, PhD, Assistant Director at the Human Immune Monitoring Shared Resource (HIMSR) at the University of Colorado Anschutz Medical Campus. HIMSR owns and operates the Vectra® 3 and Vectra® Polaris™ systems to provide multiplex immunohistochemistry services to their users.
Thanks to Akoya’s generosity, we were able to obtain temporary 30-day licenses for the inForm software to continue our analysis work remotely.
What is your research background, and what drew you to immunology?
I have worked in basic science research since my undergraduate days at the University of Missouri. I have always had an interest in cancer research, but a course in human diseases sparked my interest in immunology. I was fascinated by how complicated the immune system is, how cells can direct other cells to change their state and activity, and how these cells are so exquisitely specific for foreign invaders. Given the specificity of the immune response, I was struck by the conundrum of how immune cells can eliminate cells of self-origin in the setting of cancer. Not to age myself, but I began studying tumor immunology right as cancer immunity was emerging as an accepted phenomena – certainly well before “immunotherapy” was a standard option for cancer treatment. In fact, I was conducting post-doctoral research in human melanoma as the first immunotherapeutic targeting CTLA-4 on T cells was approved for use in melanoma patients. A truly exciting time to become a cancer immunologist, to be sure!
As I transitioned to translational research in my post-doctoral career, I became concerned about the disconnects between basic and clinical research. For example, in mouse models of cancer, we are able to deeply characterize immune cells with each research lab specializing in particular immune cell lineages and processes. In contrast, clinical researchers have limited material available and are often only able to characterize basic cell subsets; hindered by technical limitations or a lack of expertise.
To ensure that timely research opportunities were not overlooked, and that clinical researchers at the University of Colorado Anschutz Medical Campus were equipped for the considerable advances in both immunotherapies and personalized medicine, Dr. Slansky and I proposed developing an infrastructure to process clinical samples and monitor immune responses on our campus. In 2015, we were awarded a planning grant by the Strategic Infrastructure for Research Committee in the School of Medicine to determine the interest level and feasibility of establishing an immune monitoring facility on our campus. In 2016, we received the initial funding for the formation of the Human Immune Monitoring Shared Resource (HIMSR) as part of a larger institutional award to the Human Immunology and Immunotherapy Initiative. I became the Assistant Director of the HIMSR that same year, with Dr. Slansky in the Department of Immunology and Microbiology serving as the Director.
What kinds of services does the HIMSR provide?
Since its inception in 2016, the HIMSR has aimed to fill the gaps between clinical and basic science research, providing sample preparation and immune-based assays that fit the needs of our campus and scientific community. Our core services include the development of research endpoints, the selection of appropriate assays, and the testing and development of new technologies for immune monitoring. The HIMSR currently provides expertise and access to cutting-edge technologies for imaging of tissue microenvironments (Vectra 3, Vectra Polaris, and MIBI), high parameter single cell analyses by flow (15 – 30 color parameter) and mass cytometry (45 parameter), multiplex immunoassays (54-plex Mesoscale Discovery platform), and cellular assays (proliferation, cytokine production, whole blood stimulations, killing assays). We customize assays to each investigator’s needs, and provide primary data analysis services.
The Polaris has given us the flexibility to expand some of our staining panels to 9-colors and has offered the ability to generate quantifiable whole slide scans.
Why did you decide to add a Vectra 3 to your facility four years ago?
In mouse models of cancer, we are able to form a whole picture of the disease course by characterizing immune cells at several sites: in tumors, sites of metastasis, and systemically. In clinical translational research, we have very limited access to fresh tissue or biopsies for high parameter flow cytometry. However, it is well accepted that the location and nature of immune cells within the tumor microenvironment correlates with clinical outcome and, in some cases, response to treatment. Traditional immunohistochemistry (IHC) is an inefficient process that uses a lot of precious material and doesn’t allow for in-depth analyses of cells such as co-expression, proximity, and the cellular context of the tissue microenvironment. Furthermore, multiple markers are often needed to define complex immune cell subsets. We invested in the Vectra 3.0 system as a high throughput, higher parameter solution for studying tissue microenvironments and immune cell infiltrate in readily available, formalin-fixed paraffin-embedded tissue.
You recently upgraded to a Vectra Polaris – what prompted that decision and how has it affected the services you offer your users?
Despite the two-fold increase in the number of markers available on the Vectra 3 platform compared to most immunofluorescence assays, some investigators wanted to add one or two additional markers to their antibody panels. Others were concerned that sampling small areas of the tissue would bias their results and preferred to quantify immune cell infiltrate in the entire tissue slide. To meet both of these needs, we felt an investment in the Vectra Polaris would be worthwhile. So far, it has exceeded our expectations.
Although we continue to operate the Vectra 3 almost every day, the Polaris has given us the flexibility to expand some of our staining panels to 9-colors and has offered the ability to generate quantifiable whole slide scans. For users that want a “ready-made” panel and need data right away, we continue to offer our 100+ Vectra 3 7-color panels at a discounted rate. We encourage new users or those needing its added functionality to utilize our new Vectra Polaris.
How has the COVID-19 pandemic impacted your lab and users?
The pandemic has affected everyone in many ways. Our campus was officially closed to new research projects for nearly two months; only critical staff or those finishing critical experiments were allowed on campus. In the days leading up to our shut down, we stained as many slides as we could and fully loaded up our Polaris microscope. We have been able to operate the Polaris remotely, completing some of our imaging projects from home. We also had many other analysis projects to work on, so our staff has been able to work virtually full-time from home throughout this time. We were approved to partially return to campus to work on a COVID-related project in mid-April and have been slowly ramping up our research efforts since then.
Is HIMSR supporting any COVID-19 research?
Yes, the HIMSR is assisting our Cancer Center Tissue Biobanking and Histology Shared Resource to create a repository of clinical samples from patients with COVID-19. We became involved in this study after several of the immunologists in our department expressed interest in studying COVID-19 patients. The campus leadership chose to take a centralized approach to forming the biobank to ensure that clinical staff and PPE supplies are not stressed by the additional resources needed to collect these research samples. With the quick formation of an enhanced BSL2+ facility in our department, we were able to pivot from processing clinical samples for immune-related trials, most of which were on hold during the peak of the pandemic, to the cryopreservation of peripheral blood samples including plasma, PBMC, red blood cells, and nucleic acids.
These samples are being collected from patients of all ages and will be made available to researchers on campus that wish to study COVID-19. We also hope to obtain tissue biopsies from COVID-19 patients that could be studied with the Phenoptics Quantitative Pathology approach. Given the inflammatory nature of this disease, tissue microenvironments may hold significant clues to the multiple manifestations of COVID-19 and its mechanisms of severe complications.
Are you still providing data analysis services remotely?
We are performing primary data analysis for all of the assays we offer. Our staff were home for six weeks and we continue to work from home whenever possible. We had many imaging analyses projects to catch up on, and thanks to Akoya’s generosity, we were able to obtain temporary 30-day licenses for the inForm software to continue our analysis work remotely and have been able to work full-time throughout the pandemic. Having inForm available at home has also allowed several other investigators on our campus to continue working on image analysis projects as well, ensuring that their staff are also able to maintain their full-time positions. We anticipate that many publications will be completed before things return to “normal” and are excited to see the results produced during these challenging times.
Learn more about the HIMSR facility by visiting their website.
Are you interested in bringing multispectral and automated multi-slide imaging into your lab? Learn more about our Phenoptics™ quantitative pathology imaging systems here.